Petrigaba ME: A Powerful Blend for Neuropathic Pain and More
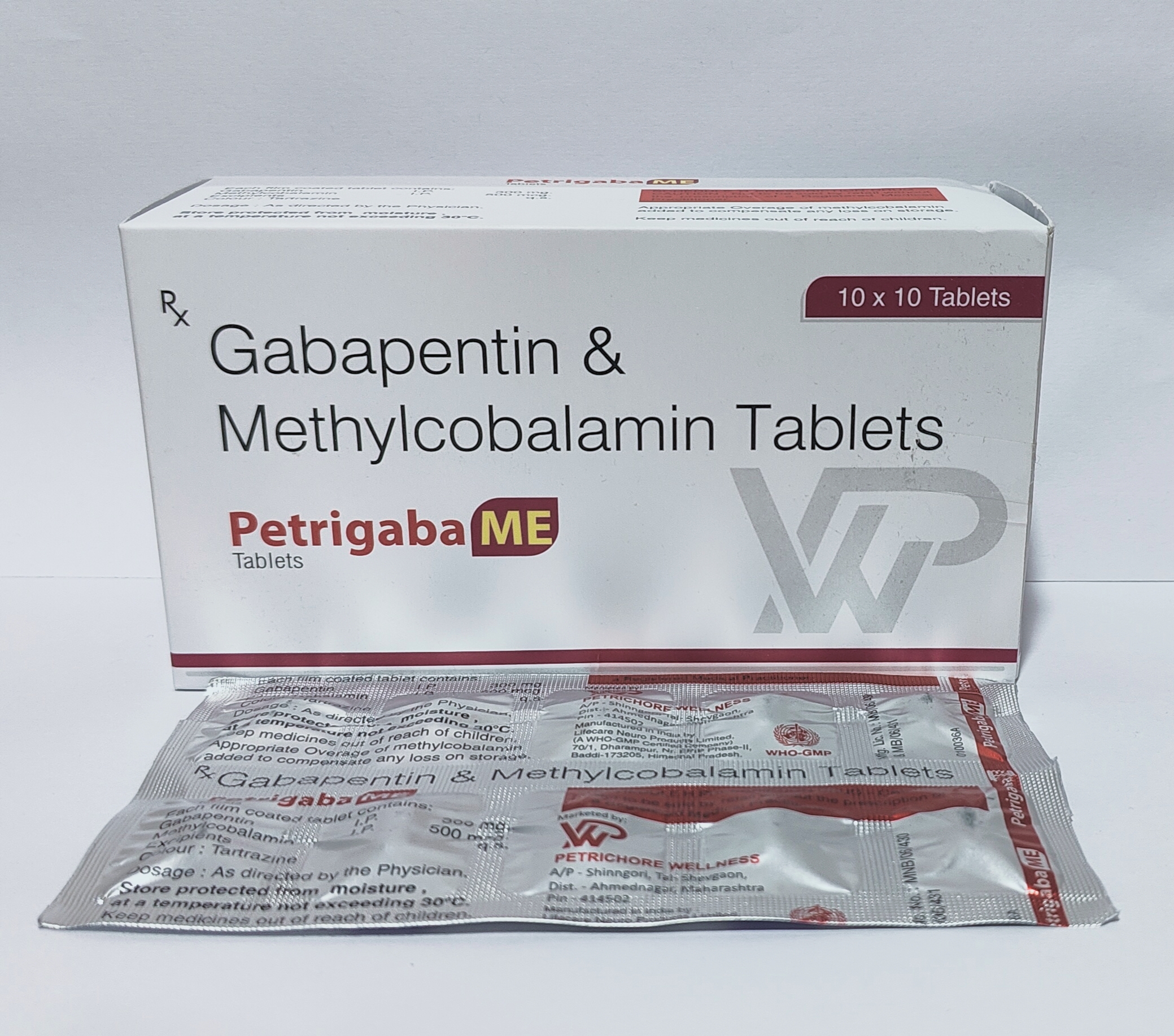
Chronic pain conditions, such as neuropathic pain, postherpetic neuralgia, and epilepsy, can significantly impact a person’s quality of life. Fortunately, medical advancements have led to the development of effective medications to manage such conditions. Petrigaba ME is one such pharmaceutical formulation that combines Gabapentin and Methylcobalamin to provide relief from these debilitating conditions. This article explores the composition, approved uses, and comparative efficacy of Gabapentin vs. Pregabalin in the context of Petrigaba ME.
Understanding Petrigaba ME:
Petrigaba ME tablets are a combination medication consisting of two key active ingredients:
- Gabapentin 300 mg: Gabapentin is an anticonvulsant medication primarily used to treat epilepsy and neuropathic pain. It works by modulating certain neurotransmitters in the brain, which helps to reduce pain signals and manage seizure activity. Gabapentin has been shown to be particularly effective in neuropathic pain associated with spinal cord injuries and other nerve-related conditions.
- Methylcobalamin 500 mcg: Methylcobalamin is a form of vitamin B12, an essential nutrient that plays a crucial role in nerve health and function. It is involved in the production of myelin, a protective sheath around nerve fibers that facilitates efficient nerve signal transmission. Methylcobalamin supplementation has been found to be beneficial in various neurological conditions and neuropathic pain management.
Approved Uses of Petrigaba ME:
- Neuropathic Pain: Petrigaba ME is approved for the management of neuropathic pain, a type of chronic pain caused by nerve damage or dysfunction. It is particularly beneficial for individuals experiencing pain associated with nerve injuries, diabetes-related nerve damage (diabetic neuropathy), and postherpetic neuralgia (a painful condition resulting from shingles).
- Postherpetic Neuralgia: Postherpetic neuralgia is a severe and persistent pain that can occur after a bout of shingles. Petrigaba ME can help alleviate the discomfort and pain associated with this condition, promoting a better quality of life for affected individuals.
- Epilepsy with Partial Onset Seizures: In addition to its use in managing neuropathic pain, Gabapentin in Petrigaba ME is also approved for epilepsy treatment. It is specifically indicated for controlling partial-onset seizures, a common type of seizure in individuals with epilepsy.
Comparing the Efficacy of Gabapentin vs. Pregabalin:
Gabapentin and Pregabalin are both anticonvulsant medications used for neuropathic pain and epilepsy management. In head-to-head studies, Gabapentin has demonstrated comparable efficacy to Pregabalin in treating neuropathic pain associated with spinal cord injury. Additionally, Gabapentin has been found to have a better safety profile compared to Pregabalin, making it a favorable choice for certain patients.
It is essential to note that while both medications have shown efficacy in neuropathic pain management, individual responses to treatment can vary. Some patients may find one medication more effective or tolerable than the other. Therefore, healthcare professionals carefully assess each patient’s unique medical history and condition to determine the most appropriate treatment option.
Conclusion:
Petrigaba ME stands as a promising medication for managing neuropathic pain, postherpetic neuralgia, and epilepsy with partial onset seizures. Combining the benefits of Gabapentin and Methylcobalamin, it provides a comprehensive approach to pain relief and nerve health support. As with any medication, individuals should consult with their healthcare providers to determine the most suitable treatment plan and to address any concerns regarding medication interactions or side effects. With proper medical guidance and the right treatment approach, Petrigaba ME offers hope for a better quality of life for those living with chronic pain and neurological conditions.
